Information Architecture as Strategic Advantage
Bill Zoellick, Senior Analyst, Gilbane Group, December, 2005
Over the past decade Siemens Medical Solutions has totally reworked its content management and information delivery system, converting it from a problem and liability into a source of competitive and strategic advantage. At the heart of Siemens’ implementation is a thorough re-architecture of its information assets. The company now manages information in the form of XML, at a very fine level of granularity. The case study provides powerful confirmation that there are substantial returns associated with investing in an information architecture that minimizes redundancy and maximizes reuse. The case study also shows readers how this large, world-wide organization achieved these goals.
You can also download a PDF version of this case study (20 pages).
Table of Contents
Introduction
Case Study Overview
Using This Case Study
Acknowledgments
In Their Own Words: Siemens Medical Solutions Perspective
Recognizing the Problem
Defining the Requirements
Selecting a Solution
Measuring Results
Next Steps
Siemens Medical Solutions Corporate Background
Success By Design
Problem Definition
System Requirements
Solution Components
Product Components and Architecture
Best Practices and Organizational Changes
A Supplier’s Voice: Astoria Software
Astoria SmartElements:
The Foundation of Siemens Medical Solutions System
Siemens Medical New Content Management Solution Highlights
About Content Technology Works
Introduction
Content Technology Works (CTW) is an industry initiative administered by The Gilbane Report to develop and share content technology best practices and success stories. The premise is that when given enough proven recipes for success, enterprise consumers will be able to adapt and replicate that success for themselves—increasing productivity and confidence. Success stories are written by The Gilbane Report analysts and are told in the voice of the enterprise adopter with final editorial control resting entirely in the hands of the adopter. The result is that:
- Vendors do not control content.
- Success stories are as opinionated and as jargon free as the adopter prefers.
- Analysis is included from The Gilbane Reportand invited contributors.
- The stories are not just about technology, but also focus on what matters to the adopter in terms of business requirements and other objectives.
Typically, this kind of valuable information is only available for purchase. CTW content is different because CTW partners subsidize the program to ensure that case study content is free. Partners want to make examples of best practices available to as many organizations as possible with the expected result that all firms involved with content technology—vendors, integrators, consultants, and technology adopters—will benefit. For more information on the CTW initiative, visit https://gilbane.com/content-technology-works-content-management-case-studies/.
Case Study Overview
For many companies, product documentation is just a cost of doing business. Siemens Medical Solutions has taken a different approach, turning its product documentation into a source of continuing competitive advantage.
Making this transition has taken a number of years, substantial investment, and attention to the documentation process and to the structure of the documentation. The return on this investment has come in Siemens’ ability to support its medical systems in 22 different languages, in its ability to provide medical practitioners with a consistent interface across its products, and in greatly improved support for the field engineers who service the equipment.
Naturally, the staff at CTW wanted to know how Siemens Medical Solutions was able to do this. What were the critical design elements in the solution? What did they learn along the way? And, finally, where do they see this investment taking them as they improve and refine their documentation system?
The very short answer to all of these questions is that the key is rigorous separation of content from layout and from the specifics of the presentation. They key to that separation, in turn, is managing the content at a very fine level of granularity.
But these short, high level answers lead to more questions. In this conversation and case study we explore the more detailed questions and learn what a company can do to enable massive reuse of content across many products and around the world in many languages.
Using This Case Study
This case study outlines the essential elements of a comprehensive approach to information reuse and content management. This is the story of one division of one world-class manufacturing company. There are, of course, elements of this story that are unique to the market that Siemens Medical Solutions serves and even to the company itself.
But there is also information here that will be useful for different kinds of organizations, serving different markets. The success that Siemens Medical Solutions has enjoyed in turning its product documentation into a source of advantage contains useful lessons for many different kinds of organizations. It provides powerful evidence that managing information at increasingly fine levels of granularity is no longer just a theoretically interesting idea, but is something that a large organization, supporting a complex product family, can put into practice.
Acknowledgments
The Gilbane Report would like to acknowledge the generous contribution of time and intellectual property from Siemens Medical Systems and from TANNER AG, the firm that assisted Siemens with system integration and with the production of documentation. Specifically, they have allocated the time of talented and heavily committed staff to improve the understanding and adoption of enterprise content technology.
In Their Own Words: Siemens Medical Solutions Perspective
CTW talked with Uwe Danner—who serves as Principal Consultant, IT-Processes at Information Systems and Key Communication Technologies for Siemens Medical Solutions—about the design and implementation of the company’s new approach to managing product documentation. Mr. Danner was joined by Markus Deisböck and Alexander Witzigmann of TANNER AG, who spoke to some of the implementation details. Excerpts from this conversation provide a useful introduction to the issues that Siemens Medical Solutions chose to address, as well as to the approach that the company followed in developing the new system.
Our conversation with the Siemens team focused on the key tasks facing any company that makes a transition to a new content management system: recognizing the problem, defining the requirements, selecting a solution, and evaluating results. We have organized these excerpts from our conversation according to these key tasks. The questions that we asked are presented in italic text; the answers from the Siemens team are indented.
Recognizing the Problem
What motivated the change from your existing documentation management system to a new, more comprehensive approach to content management?
Uwe Danner: “We ran into a number of different problems. We had trouble with the traceability of our documents. We also ran into trouble because of new regulations from the FDA that were difficult to comply with under our old system. Speaking more generally, we just had lot of information, and nobody could handle all of this information in the right way.”
“We had a bunch of different systems. One critical system, which was called Global-U, was at the end of its technical possibilities. So we had to change from Global-U to something else. Unfortunately, we found that it was not possible to migrate the data from this old system to a new one. We had to convert thousands and thousands of pages. So, we knew that we had to make sure that, in the new system, the information would be independent of the system.”
Defining the Requirements
What did the new system need to able to do that the old one could not?
Uwe Danner: “One thing, that I already mentioned, was that we needed to make sure that our information would continue to be useful and available as we developed new systems. So, back at the time we were doing this, we decided that the information would need to be captured in SGML, which is an ISO standard and guaranteed that the information would be useful over a long time.”
“For our customer service, we needed up-to-date information in the field. This not only had to be up-to-date, but also complete. It needed to include installation guides, maintenance guides, service guides, and lists of spare parts. All of this needed to be available in a single set of computer-based documentation – which we call CB-DOCs – with all the information cross-referenced internally.”
Markus Deisböck: “There are actually two groups of users, and also two groups of requirements. One is the customer services group with all the field engineers. The other is the group that develops the product and so needs to develop manuals for the end users.”
Uwe Danner: “Yes. And with this other group the requirement was to save time by being able to reuse information in the process of translation.
“You should understand that for the business units and operating managers the effort required to produce end-user documentation is substantial, because of the quality checks built into the process. These are medical devices, and the information absolutely must be correct. Then, on top of that, we have had increasing customer demand for delivery of all operating manuals in the language of the country where the product is used. We increased the number of languages that we support for operating manuals from 4 languages to 22 languages. We need to be able to deliver operating documentation in all of these languages at the same time that we deliver the product.”
How did the new content management system help with managing the translation requirements?
Alexander Witzigmann: “Moving to a system that provides element level addressability and versioning allows us implement language variant management at the element level. That means that if information within a particular element changes, only that specific information needs to be translated into all the languages that we support. This is the main advantage that we get from managing information at a very fine level of granularity. Only the new, changed information needs to be sent through the translation process and all the other information is left untouched. This is not only a matter of translation cost, but also is a matter of quality assurance and time to market.”
It sounds like element-level addressing emerged as a key requirement. Were there other advantages gained from element-level addressing?
Alexander Witzigmann: The main advantage apart from translation is that you have the flexibility to change the level of reuse granularity without having to change the data model.
You have raised an interesting issue. Have you changed the DTDs over time?
Uwe Danner: “Of course. We have made several changes over the years.”
Was providing this flexibility an important consideration?
Alexander Witzigmann: “Yes, it is very important. With the system we selected, the data model is driven from the XML/SGML infoset and not on some other system, such as a relational database. So, the big advantage here is that you can just import a DTD and then begin using it. You do not have any additional configuration work. You do not need a database administrator to make changes.
Were there other requirements?
Uwe Danner: “One of the big requirements was to have less information on paper and more on the machine. We have done this using a common platform and single internal operating system across all the medical devices.”
Alexander Witzigmann: “It is very important that the look and feel across all the different Siemens medical products is the same.”
Uwe Danner: “And, also, in terms of basic functionality, they are the same, and work in the same way. They have the same software. So, for a technician servicing the machine, he is dealing with the same system. For the user, although there are customizations for the different products, they find that they operate in the same way.”
Markus Deisböck: “So, imagine a nurse is working with an MR [Magnetic Resonance] product, and then, an hour later, she has to work with a CT [Computed Tomography] product, for example. It is very helpful to have the same look and feel, the same expressions, and the same kind of operation and workflow of operations across the different devices.”
Selecting a Solution
How did these requirements shape your choice of a solution?
Uwe Danner: “We began by looking at eleven different document management systems. In the end, the choice came down to two different solutions. For one of the systems, the advantage was that it had integrated workflow. The disadvantage of this system was that it did not include full integration of SGML. In particular this system would not have allowed us to handle SGML at the element level.”
“The other system offered full ability to handle SGML at the element level, but did not offer integrated workflow. The ability to handle SGML at the element level was the key requirement, so we went with this system. We decided that we would be able to address the workflow requirement in the future by bringing in the workflow capabilities of our SAP system.”
“We made this decision six years ago. Since that time we have continued to look at competing products as we go to trade shows and, over that time, have become increasingly sure that we made the right choice.”
Measuring Results
Have you been able to quantify results? Do you know that you are meeting your objectives?
Uwe Danner: “It is very difficult to measure the results of individual parts of the system because there are many factors and many interrelated objectives. I have already mentioned our goal of having less information on paper on more available on online help. That changed many things about the process.”
“A different factor is our emphasis on using more pictures and less text. Capturing and adding good pictures takes time. So, while we are improving efficiency and reducing time in some areas, we are adding new work in others.”
“Or, take translation for example. We have started using a translation management system, but also use our content management system to improve the translation process. The systems are used together. It is impossible to know how much advantage comes from one system and how much from the other.”
I see. So, the improvements in production time are difficult to measure because you have also used the new content management system to expand the quality and quantity of the documentation that you deliver. I would assume that these changes have an impact on what happens in the field …
Uwe Danner: “We have about 3,900 service engineers. For every product we provide them with computer based information. To keep it up to date we support a data download of the changed information – enabled by our element-level change control. Service engineers download updates before going out on a service call.”
“We have the same CMS worldwide. In Europe we have a server in Germany. We have one server in Shanghai, and one server in California.”
Andrew Witzigmann: “Having a worldwide system is essential to harmonize the information delivered to the field. Each service engineer, whether he is in China, Europe, or the States, gets the same information, structured in the same way.”
Markus Deisböck: “The point is that Siemens is now able to hit the right solution for most of the support cases the first time. The field engineer is able to find the right solution the first time he looks for it. This saves money and keeps the customer happy. The rate of getting the right solution the first time is now up over 70%.”
Next Steps
Where is Siemens Medical Solutions taking this investment and new system as you look ahead over the next few years?
Uwe Danner: “Every year we have a lot of new requirements. We can improve it by adding more web-client access. We can improve it by adding workflow to automate more of the processing. For example, we can add workflow capabilities to automate the interaction between the CMS and the translation management software.” Next Steps
“We also have new requirements for online help. Our goal is to have more information in the medical product and less in additional documentation that is shipped with the product.”
“As we continue to use the system we develop new requirements every year, and then have to prioritize them.”
Andrew Witzigmann: “The overall strategy is to bring the documentation more closely into the product.”
Give me an example of what it means to bring the documentation into the product.
Uwe Danner: “We sell diagnostic machines, for example. Every product from Siemens Medical Solutions has integrated software to support the operating interface. We could, for example, have interactive online help.”
Markus Deisböck: “You don’t need this information in the manuals. You need it on site, so that is there when people need it.”
Andrew Witzigmann: “One approach is that the information itself can drive the product. For instance, in one project, the operating workflow comes from the documentation. So the documentation actually drives how the product interacts with the user. That’s really what the future is.”
“So, it’s not only information, it is also interaction. The information not only tells you what you have to do, but you can also do it, all in the same interface. So, if the machine tells you ‘Please adjust this value,’ you can make that adjustment right in the same place. The information and the operation come together.”
Is the XML foundation for your information part of what makes this possible?
Uwe Danner: “Yes. Absolutely. The product uses the XML natively.”
Andrew Witzigmann: “The publishing channel for this kind of product is XML, with the Siemens product using the XML.”
Uwe Danner: “We don’t have all this in place yet, except for a few projects. But this is where the future is.”
Siemens Medical Solutions Corporate Background
Siemens Medical Solutions offers imaging equipment, information technology, management consulting and services to hospitals, clinics, medical laboratories, and other healthcare businesses. The company’s products and solutions address medical needs in a wide variety of medical applications, including angiography, computed tomography, fluoroscopy, magnetic resonance imaging, mammography, nuclear medicine, oncology, patient monitoring, radiography, surgery, ultrasound, urology, and ventilation and anesthesia.
The company’s website describes its product differentiation in terms of “exceptional levels of connectivity and information integration.”
Siemens Medical Solutions is a business segment of Siemens AG. 2005 revenues were just under €7.6 billion, producing a group profit of just over €976 million. The company has headquarters in Malvern, Pennsylvania and Erlangen, Germany.
For more information on Siemens Medical Solutions see http://www.medical.siemens.com/.
Success by Design
Siemens’ successful deployment of a next-generation content management system provides useful insights for other companies seeking more from their CMS investments. What may be the most important lesson from the Siemens experience is that the company’s success traces back to an early decision that is so large and pervasive that it would actually be easy to not notice it. From the outset, Siemens decided that delivering information was a strategic issue—not just a product support issue—and therefore was potentially a way for the company to differentiate itself in the medical systems marketplace.
Here is what Siemens Medical Solutions has to say about its products on the company website—the emphasis is theirs, on the website, and not something that we have added.
Siemens clinical and information systems are known for their outstanding performance, speed and ease of use. Our product line offers exceptional levels of connectivity and information integration, to bring you the clinical information you need, where and when you want it. The result: streamlined workflow and better patient care.
Put another way, Siemens Medical Solutions understands that offering cutting edge engineering and technology in its products is a requirement that places the company in the top tier of medical technology providers, but also realizes that excellent engineering, alone, cannot give the company significant advantage over the other first-rate competitors. The key insight was that, for the doctor or nurse using the system, technology is not the issue. In the final analysis, the purpose of world-class medical technology is that it enables better treatment decisions. In short, medical solutions are built on the collection, presentation, and use of information.
This understanding necessarily changed the way that Siemens approached the replacement of its old approach to product documentation and support. The company was no longer merely selling and supporting equipment. It was selling information and decision systems. The implication was that Siemens needed to build an internal capability to enable delivery of information in new, more useful ways.
Problem Definition
The excerpts from the interview with managers from Siemens and TANNER show that Siemens understood that it was addressing a range of problems when it went shopping for a new CMS. Some of the problems were near term and the result of new regulations. For example, the company knew that the FDA’s rules for internal control of information in 21 CFR Part 11 would require change control and audit tracing capabilities that were beyond the functionality of the document management and production capabilities that the company had in place in the mid-1990s.
Looking a few years further out, Siemens Medical Solutions saw that it would be clearly advantageous to support a greater number of languages in its operating instructions and end-user interface. So, more cost-effective, accurate approaches to managing translation became an important part of the problem definition.
The company also wanted to provide better, more complete, more up-to-the minute support for its field service engineers. For one thing, this meant moving away from paper manuals to an information delivery that would support interactive queries and hyperlinks. It also meant creating an information architecture that could support fast updates as service information changed and improved with time.
The conversations with the Siemens and TANNER personnel also reveal a scope of problem definition that moved well beyond immediate concerns, reaching out to the strategic insight that medical systems are really information systems and decision systems. As Alexander Witzigmann put it: “it’s not only information, it is also interaction. The information not only tells you what you have to do, but you can also do it, all in the same interface. So, if the machine tells you ‘Please adjust this value,’ you can make that adjustment right in the same place. The information and the operation come together.”
System Requirements
Although these problem definitions span different ranges, from near term to future, they all pointed to certain core requirements.
Foremost among these requirements was the need to separate content from presentation. In practice, at many other companies, this separation is honored more in theory than in practice. We have learned over the years that really keeping content independent from presentation can be notoriously difficult. Siemens, with its desire to use the information to support many different functions, for different users, across different delivery mechanisms, knew that they would need to be rigorous in maintaining this distinction.
Equally important was the need to handle information at a fine level of granularity. Fine granularity would help make the rigorous separation of content and presentation possible. It would also minimize the cost of managing changes to documentation and procedures in an environment where every change must be translated into more than twenty different languages. It would improve quality and accuracy, by minimizing the scope of any change, and would bring down the cost of keeping the systems on the laptops used by service engineers up-to-date.
Back in the mid-to-late 1990s, when Siemens Medical Solutions was planning this new system, the requirement for rigorous separation of content and presentation implied use of an SGML solution. As Uwe Danner noted in the interview, the experience of finding its information “locked up” in an earlier, proprietary format also argued for use of an ISO standard such as SGML. As the system, the technology, and the market have evolved over the past six years, that initial focus on SGML has migrated to use of XML.
Given the commitment to use of SGML, and, later, XML, Siemens recognized that it needed a content management system that handled SGML and XML natively. The company knew from experience that its information architecture would grow and change with time, as the company developed new products and new uses for the information about how to service and use these products. Siemens knew that it would be difficult and expensive to manage this growth and change in a system where the content structure was expressed using a relational database, rather than directly, through the SGML/XML infoset.
As Uwe Danner explained in the interview, the need to manage information natively in SGML or XML, at a fine level of granularity, narrowed the company’s choice of a CMS supplier to one vendor. That vendor was Astoria Software.
Solution Components
The Siemens Medical Solutions authoring, content management, and publishing system is complex, since it supports authoring from a variety of sources, translation management, and output in a wide variety of presentation formats and languages. At the center of the system is Astoria Software’s XML based content management, running on an object database. As noted in the interview, the underlying object database enables direct use of the XML infoset to control the information architecture.
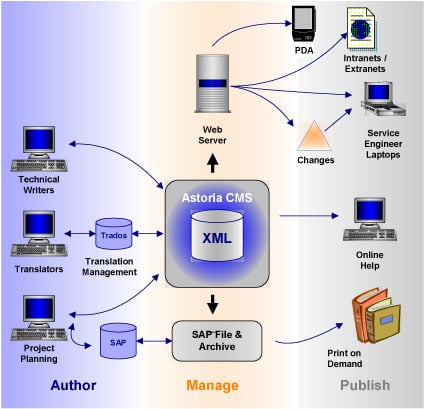
Product Components and Architecture
Figure 1: Siemens Medical Solutions system architecture
Most of the authoring is done with Adobe FrameMaker, using Astoria’s FrameMaker integration package. The translation management operation combines the element level management offered by Astoria with the translation memory and other tools offered by Trados. As noted in the interview, workflow for the print-on-demand operations makes use of SAP’s project planning tools.
This system architecture diagram could be expanded to the right, to include the actual Siemens Medical equipment—the X-Ray machines, CT scanners, and so on—as part of the information architecture, since these devices are actually the delivery vehicle for the online documentation. As noted in the interview, Siemens is now designing its medical devices to make native use of XML to control the user interface and present instructions and help to the user.
Best Practices and Organizational Changes
Developing and supporting a fine-grained information architecture without information redundancy requires more than technology. Creating this kind of system requires new approaches to authoring and more effective and extensive collaboration between writers, human-factors designers, and engineers. Without such collaboration, you still end up saying the same thing in different ways, in different places, despite all the technology investment.
Before moving to the new, XML-based solution, Siemens Medical Solutions was creating documentation using a variety of approaches, some just based on word processing tools such as Microsoft Word. Creation of service documentation was often separated from creation of end-user documentation.
Pulling these different activities together into a single, well-integrated system required careful analysis of where information came from and how it was used. Siemens engaged TANNER AG to assist with this information architecture design work. The initial design effort took the better part of a year.
This is an important point to emphasize for other companies hoping to build their own, highly integrated, highly granular systems. Siemens did not approach this project as a set of incremental changes to their existing system. Instead, they approached the problem as a new design and implementation, working from the ground up. They began by identifying the two key groups of users—service personnel on the one hand and medical personnel on the other—and focused on questions such as “What do these users need to know?” and “What do they need to do?”
Much of the success of the Siemens Medical Solutions system can be traced to this willingness to start with a clean sheet of paper and build the system that they needed, rather than trying to improve or extend an existing system.
Not surprisingly, adoption of such a different approach to creating, managing, and publishing information resulted in new ways of working within Siemens Medical Solutions. Service specialists now work more closely with design engineers. Customer support personnel and human factors specialists are now part of the design process as well, a collaboration that is made more fruitful by the ability update the user-interface on products in the field through the delivery of new information in XML.
All of this collaboration is, of course, enabled by technology. But the result of the collaboration reaches well beyond the technology, creating new knowledge and greater responsiveness to customer needs throughout the company.
Results
As Uwe Danner explained in our interview, it is difficult to pinpoint particular outcomes and to connect them with particular design decisions or technologies within the Siemens Medical Solutions content management system, since Siemens changed so many different things as it moved from its old approach to the new one. With so many changes happening at once, it is nearly impossible to connect particular effects to specific causes.
It is, however, possible to look at results as a whole.
- Siemens Medical Solutions is currently providing up-to-date documentation and online help to 60,000 customers around the world.
- The company is supporting more than 190,000 product installations across this customer base.
- Customer documentation is supported in 22 languages.
- This support requires about 15,000 translations a year.
- The company produces approximately 100,000 new pages of documentation a year.
- There are 150 technical authors, worldwide, producing this information.
- The documentation supports the work of 3,900 service engineers.
- Service engineers are able to find what they need on their first try more than 70% of the time, resulting in an increase in “First Time Fix Rate” (FTFR).
- The improved FTFR has resulted in a decrease in “Mean Time To Repair” (MTTR).
Apart from these particulars, there are also the broader outcomes associated with the Siemens’ decision to make information delivery a part of the company’s strategic vision. Now that Siemens Medical Solutions has successfully constructed a highly granular, XML-based content repository, it is positioned to intensify its focus on the medical professional’s use of information. With the addition of native XML support its medical equipment, Siemens is positioned to deliver “exceptional levels of connectivity and information integration,” providing doctors and nurses with “the clinical information they need, where and when they want it.”
A Supplier’s Voice: Astoria Software
Siemens Medical Solutions is an industry-leader in the global manufacturing of complex technical products and services for healthcare. To meet Siemens’ objective of building a new information management and publishing system to efficiently deliver consistent product information for customers and service technicians, Siemens looked to Astoria Software and its consulting and systems integration partner, TANNER, AG.
It was clear that Siemens Medical’s business plans required a solution that could manage the heavy demands of publishing, delivering and then updating thousands of technical product documents published in multiple languages and formats. Not only did Siemens need a new system to improve efficiencies and reduce cost of its annual publishing, it also had a vision of using this information strategically to contribute to top-line objectives in the form of information delivered as added value within its products to support the decision-making process of its end-user healthcare providers.
It became clear early to Siemens and TANNER that Astoria Software’s XML content management solution was uniquely qualified to deliver on solution objectives and would best serve as the foundation for the new publishing infrastructure. As the most mature, robust and flexible technology solution available for managing XML content and documents, Astoria allows Siemens a common system to support all its maintenance and product operation documentation.
Astoria SmartElements: The Foundation of Siemens Medical Solutions System
Integral to the Siemens solution is Astoria SmartElements, an Astoria Software feature unique in the industry in enabling management of individual content components at the most granular level, instead of managing content within whole documents or as large chunks both common to other solutions. The innate advantage of Astoria SmartElements delivers Siemens benefits throughout its complex author-to-publish process; content components are easily managed, stored, searched, updated, re-used, sent for review via Astoria’s automated workflow, sent for translation, then assembled and published. The flexibility and control of Astoria SmartElements ensures any current, or earlier version of an element can be re-used throughout its lifetime, with complete audit trail.
Astoria SmartElements has changed the nature of Siemens multi-language publishing business. To update its documents in 22 different languages, they can manage the costs of translation, with only the content that has been changed sent through the translation process. With the Astoria solution managing the content independently from its document structure, or schema, its content is “format neutral,” enabling the easy, simultaneous output to multiple delivery channels – print or electronic.
For Siemens, its Astoria-based content management and publishing solution has had a significant positive impact on their publishing processes, enabling them to meet the requirements set for the project, and capitalize on their new investment.
Siemens Medical New Content Management Solution Highlights
- Information Consistency: With Astoria, Siemens is able to deliver consistent information across business groups—with the sharing of re-used content between product documentation and customer support/maintenance publications. An ability to re-use content resulted in productivity increases of up to 60% in authoring and revisions on documentation
- Translation Cost / Time Savings: Siemens reduced its translation budget by more than 75% and improved time-to-publish updates in local languages. Astoria SmartElements in concert with SDL/Trados translation management software ensured that only updated content would be sent out for translations, versus all content in a document.
- Content Quality/Accuracy: Siemens now has a means to track the accuracy and quality of its content, improving access control to those responsible for publishing and approving updates, and tracking the change history of content over its lifetime, with audit trail and reporting capabilities.
- Multi-Channel Publishing: Siemens now publishes 90% less content in print format with its move to Astoria for digital content management. It can deliver publication updates to end-user customers, and customer service engineers faster, more accurately, and in electronic and print formats with equal ease.
With the success of its product information publishing project based on Astoria Software, Siemens Medical Solutions is now more efficiently and cost-effectively publishing accurate, quality, consistent documentation that reinforces its worldwide reputation for product excellence, with an eye toward even further improving future product offerings with addition of high-value information.
Conclusions
From our standpoint within the Gilbane organization, part of what makes the Siemens Medical Solutions story so useful and exciting is that it shows what a company can do if it takes its information architecture seriously. For years we have known that content should be rigorously separated from presentation, that information should be managed at a fine level of granularity, and that information should be maintained in only one instance in the repository, being reused rather than repeated. The first substantial efforts to put these precepts into practice date back to the late 1980s and the CALS SGML initiative undertaken by the U.S. Department of Defense.
Despite the long pedigree of these ideas and precepts, we don’t often see them rigorously, thoroughly put into practice. Often, information is still managed more at the chapter or section level than at the paragraph or sentence level. Often, it is simply easier to say something in a different way, for a different purpose, than to find and reuse existing information.
Siemens Medical Solutions has shown that taking the more rigorous approach is completely doable. Just as important, Siemens demonstrates that there are substantial rewards available from element level management and full separation of content and presentation.
Siemens success and accelerating momentum in this area raise some important questions: “What makes Siemens Medical Solutions different? How are they able to get this right when others tend to settle for more of a half-way effort?”
There cannot be hard, precise answers to such questions, but we can make some observations which might be useful for organizations considering a renewal of their own content management systems:
- Siemens Medical Solutions decided that the ability to manage and present information was a matter of strategic importance. From this key decision followed a number of important factors contributing to success, such as a commitment to careful design.
- The new system was a “from the ground upward” replacement of the older system, not a set of incremental modifications to a fundamentally flawed design.
- The design focused on user objectives and needs, not on internal constraints.
- The need to support information delivery in nearly two dozen languages helped the company stay focused on the importance of fine-grained content management.
- Siemens Medical Solutions selected a content management technology platform that enabled them to actually achieve the company’s ambitious goals, avoiding the conflict and cost that arises when there is a poor fit between technology platform and objectives.
This list of observations is an immediately useful outcome from Siemens’ work and from this case study. There are also some outcomes that are less immediate, but that could be just as important and useful.
In particular, it will be interesting to watch Siemens Medical Solutions as it moves forward into the next stage of its integration of information and products. For twenty years the Gilbane team has been sensitive to the potential to blur the distinction between “documentation” and “product,” marrying the two concepts to create a more useful, responsive product. In many applications, unfortunately, such integration has not gone much beyond simply delivering the documentation electronically, within the product, in the form of online help. Online help is a wonderful thing, but it leaves the distinction between documentation and product intact.
Siemens is moving in the direction of a true marriage of product performance and product information, using XML-based product information to drive the XML-aware medical device as it responds to what the user is doing.
It is not surprising that this kind of development is taking shape in the medical technology market—these are, after all, very high value products that integrate enormous amounts of information to present a useful picture of a patient’s condition to a highly trained professional. So, as early markets go, this one is nearly ideal for the introduction of the kind of cutting edge use of information that Siemens is pioneering.
What should be interesting, as Siemens Medical Solutions succeeds in this initiative, will be the innovations that Siemens brings to market in the way of managing and creating this marriage of device and documentation. These developments will be potentially useful in other high value, specialized technology products and, perhaps over time, in more routine interactions between products and users.
This case study, then, presents at least two kinds of useful insight. The first consists of a look at how one company has managed to implement a highly granular, highly flexible content management system in a complex environment—coupled with a look at the benefits that come from such an implementation. The second insight looks downstream, at the strategic potential of having such a system in place as a foundation to build upon.
About Content Technology Works
When we first conceived of an initiative that would develop and distribute success stories that placed recipe over ingredients and favored no supplier, technology or computing standard, we also recognized that our most significant hurdle would be to recruit vendors to subsidize such an independent and open process.
Since the CTW program was first conceived in late 2003, we have sought out suppliers who were passionate about and committed to content technology as a game-changing force in the markets that they serve. Our CTW partners know that public, open and unfettered access to successful enterprise deployments, regardless of the technology mix, only benefit the commercial aspirations of organizations that offer material, dependable and predictable value.
The Gilbane Report team wishes to thank these diverse and often competing organizations for their generous support and sponsorship of the development, promotion and distribution of CTW material. They are: Software AG(TECdax:SOW), Sun Microsystems (NASDAQ:SUNW), Artesia Digital Media, a Division of Open Text, Astoria Software, ClearStory Systems (OTCBB:INSS), Context Media, Convera (NASDAQ:CNVR), IBM (NYSE:IBM), Idiom, Mark Logic, Open Text Corporation (NASDAQ:OTEX), SDL International (London Stock Exchange:SDL), Vasont Systems, Vignette (NASDAQ:VGN), and WebSideStory (NASDAQ:WSSI).